What we know about Descovy’s impact on weight & cholesterol
Tenofovir alafenamide (TAF), co-formulated with emtricitabine in the combination pill Descovy (F/TAF) is the newest option for once-daily PrEP for people who may be exposed to HIV through anal sex. The medication joins F/TDF (tenofovir disoproxil fumarate with emtricitabine, brand name Truvada) as one of two medications approved by the FDA for HIV prevention as PrEP.
Because TAF concentrates at lower levels in the blood than TDF, Descovy has been found to have less of an impact on bone mineral density and kidney function than Truvada. (For the majority of people, Truvada is safe to take and causes no clinically significant bone or kidney changes.)
This benefit of Descovy may be offset by its potential impact on weight and lipid levels, and some in the HIV community have raised concerns about these metabolic changes caused by TAF.
“When we look at TAF versus TDF, a lot of people aren’t aware that TAF actually is different than TDF. It causes greater rises in body weight, greater rises in lipids, glucose and potentially a higher risk of diabetes,” said Andrew Hill, PhD, from the University of Liverpoolin a webinar by AVAC. “It’s not clear whether TAF has a better or worse safety profile than TDF.”
“Our experience in HIV care is that we’ve been using [medications containing TAF] pretty consistently for over four years now in clinical practice and there haven’t been any widespread safety concerns,” said Neal Sheran, MD, an HIV specialist from Mission Neighborhood Health Center in San Francisco. “But the truth is, is that sometimes it takes 10 to 20 years to see toxicities of these HIV medications. Now we’re finding out things like there may be higher weight gain with TAF. What might that look like in the future? Is it going to mean people having higher blood pressure, higher coronary artery disease or higher risk of diabetes?”
There is some evidence from clinical trials that TAF may cause weight gain and worse lipid profiles in people taking medications for PrEP or HIV treatment. Here’s a breakdown of what we know from HIV treatment and PrEP studies.
Higher weight gain with TAF
When used by people who are HIV-negative for PrEP, Descovy may cause a small amount of weight gain—or at least may not prevent normal weight gain as happens with Truvada. This is the case for cisgender men and possibly trans women (Descovy has not been studied for PrEP in transmasculine people or cisgender women).
On average, cis men and trans women who participated in the DISCOVER study of Descovy for PrEP gained 1 kg (2.2 lbs) after taking medication for 48 weeks. People taking Truvada lost a small amount of weight at weeks 12 and 24, and then returned to baseline weight at weeks 36 and 48.
In the metabolic sub-study of the iPrEx study, cis men and trans women taking Truvada for PrEP experienced weight loss after 24 weeks, with weight increases at later visits similar to that of placebo. The average total weight loss among people taking Truvada compared to people taking placebo was -0.8% at week 24. By week 96, the median weight of people taking Truvada was not significantly different from placebo.
Modest weight gain is expected in people during adulthood. Because people taking Truvada decreased their weight by -0.1% at week 24 in the iPrEx study, and showed less weight gain than people taking placebo at weeks 48 and 72, Truvada may initially prevent normal weight gain but, according to this study, this effect may fade with long-term use.
The bulk of the information we have about TAF and TDF’s effects on weight comes from studies with people living with HIV. Because it is recommended that TAF and TDF are always used in combination with other HIV medications, and because HIV itself can change body weight, these differences can be more difficult to parse. Even still, a range of studies have found that people experience a greater rise in body weight when their HIV regimen includes TAF compared to TDF.
The ADVANCE trial, for instance, found that people living with HIV in South Africa who took dolutegravir (an integrase inhibitor), emtricitabine and TAF gained significantly more weight over 96 weeks than people who took the same regimen with TDF instead of TAF. People taking a regimen that did not include dolutegravir (efavirenz with F/TDF) gained the least amount of weight. Over time, women gained more than men, and weight gain continued to rise over time without plateauing over the 96 weeks.
In the study, men on the TAF-containing regimen gained an average of 5 kg (11 lbs) over 96 weeks, those taking the TDF-containing regimen gained 4 kg (8.8 lbs), and those taking the efavirenz regimen gained 1 kg (2.2 lbs). Women on the TAF-containing regimen gained an average of 10 kg (22 lbs), 5 kg (11 lbs) on the TDF-containing regimen and 3 kg (6.6 lbs) on the efavirenz regimen.
Andrew Hill, in an analysis presented at the European AIDS Conference in November 2019, said that the difference in weight gain seen between men and women in the ADVANCE study may be due to sex differences in the metabolism of the drugs.
Mean change in weight (kg) to Week 96: men
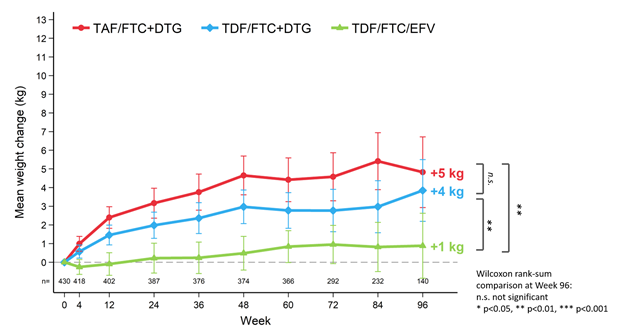
(Slide: W. Venter and colleagues, IAS 2019, Abstract WEAB0405LB)
Reporting the 48-week outcomes from the ADVANCE study, Willem Venter, PhD and colleagues note that the weight gain that occurred in the study is “unlikely” to be a return-to-health effect, since viral suppression, CD4 recovery and adverse events were similar across the three groups.
“Use of TDF has been associated with smaller increases in weight than TAF or other NRTI combinations; however, it is unclear whether TAF amplifies or TDF mitigates the weight-gain effect of DTG [dolutegravir],” the authors noted.
“This information is something relatively new that wasn’t on our radar until fairly recently,” said Sheran. “There can be weight gain with HIV treatment, and there is higher weight gain associated with certain antiretrovirals. It does look like there’s a weight gain difference between TDF and TAF, with higher weight gain for people taking TAF.”
Rise in lipid levels with TAF
There are a number of studies showing that TDF-containing regimens oftentimes improve lipid levels by decreasing total cholesterol, LDL (“bad”) cholesterol and triglycerides, while TAF-containing regimens increase these levels. Higher levels of total cholesterol and LDL cholesterol can promote plaque buildup in arteries, which can lead to coronary artery disease, stroke and other health problems.
In the DISCOVER study, which compared Descovy (which contains TAF) to Truvada (which contains TDF) for PrEP, triglyceride and LDL cholesterol levels increased at 48 weeks for people taking Descovy compared to no change or a decrease in people taking Truvada. Decreases were seen in total cholesterol and HDL cholesterol (although decreases were smaller for people on Descovy versus Truvada).
Other studies have compared the lipid levels of people living with HIV taking TAF-containing or TDF-containing treatment regimens or switching from a TDF-containing regimen to a TAF-containing regimen.
An observational study conducted in Spain compared the lipid profiles of people on Genvoya (elvitegravir, cobicistat, emtricitabine and TAF) or Stribild (elvitegravir, cobicistat, emtricitabine and TDF) for HIV treatment over 48 weeks.
Lipid levels (total cholesterol, LDL and triglycerides) increased from baseline among people taking Genvoya (the TAF-containing combination medication) while remaining stable among people taking Stribild after 48 weeks of treatment. A higher percentage of people taking Stribild also had total cholesterol levels (40%) and LDL levels (30%) out of range after 48 weeks of treatment. Six people in the study discontinued Genvoya due to changes in lipid levels, and lipid levels returned to normal levels after returning to treatment that included TDF.
“We observed an increase in lipid levels, a significant increase in TC:HDL‐C (associated with cardiovascular disease risk) and an increase in lipid‐lowering prescription, which could give rise to an increase in cardiovascular risk,” said Purificación Cid Silva and colleagues. “This study provides the first experience in real clinical practice regarding lipid profile alterations in patients being treated with EVG/c/FTC/TAF. Our findings show that this fixed combination had a negative influence on the lipid profile after 48 weeks’ treatment, both in naïve and in experienced patients. This worsening of the lipid parameters has not been observed during the treatment with the EVG/c/FTC combination with tenofovir disoproxil fumarate.”
Other studies have found that people living with HIV experience significant increases in total cholesterol, LDL and triglycerides when they switch from a TDF-containing regimen to a TAF-containing regimen.
In a study with more than 4,000 adults living with HIV in the U.S., switching from a regimen containing TDF to TAF was associated with worsening lipid levels. Lipids increased on average by 9% for total cholesterol, 12% for LDL, 8% for HDL and 26% for triglycerides when compared six months prior to switch to one week or more after switch. Study participants were taking protease inhibitor, NNRTI and INSTI-based regimens, and findings do not appear to be driven by boosting agents.
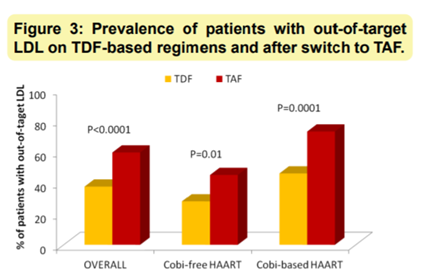
A smaller study, by Lidia Gazzola and colleagues, with 221 people living with HIV found that the boosting agent cobicistat further increased the likelihood that people switching from a TDF-containing regimen to a TAF-containing regimen would experience an out-of-range LDL lab value. (The quad combo pills Stribild and Genvoya both contain the boosting agent cobicistat.) People switching to TAF in cobicistat-free regimens had double the risk of out-of-target LDL; with cobicistat, the risk increased to 3.1.
For people taking TAF, lipid changes need to be evaluated for possible clinical significance by a health care provider, said Chris Hall, MD. Although changes may be less consequential for young, otherwise healthy people, people with pre-existing conditions such as diabetes or high blood pressure may be more affected.
Switching from TDF to TAF
Overall, Sheran said that it’s helpful to think of the option to switch from TDF to TAF (or to choose one type of regimen over the other) as a choice guided by input from a provider.
“My opinion in terms of prescribing is to be patient-centered,” he said. “TAF is another option, and there doesn’t have to be dogmatic black and white rules about every aspect of medication choice. There are compelling reasons to switch to TAF-containing regimens if people have underlying kidney or bone issues. I do think there is a pharmaceutical industry influence, so we will see people switching over to Descovy for PrEP. But for many people, there may not be a compelling reason to switch. It is good that we have another option for people, so if they don’t tolerate Truvada there is another option.”
—
Medical review by Neal Sheran, MD
Free PrEP services at San Francisco AIDS Foundation
SFAF offers daily PrEP and PrEP 2-1-1 dosing options. Make an appointment.
Want to be connected to PrEP, but live outside of the San Francisco Bay Area? Find a PrEP provider near you.
References
AVAC, TAG, WRI and The Well Project. Advocates’ Debrief on the Science of Daily F/TAF vs TDF/FTC as PrEP. Webinar, 2019.
Cid-Silva and colleagues. Treatment with tenofovir alafenamide fumarate worsens the lipid profile of HIV‐infected patients versus treatment with tenofovir disoproxil fumarate, each coformulated with elvitegravir, cobicistat, and emtricitabine. BCPT, 2018.
Gazzola, L. and colleagues. Increases in lipid profile after switch from TDF to TAF-based HAART regimens in a cohort of HIV positive patients: Is it clinically relevant?. HIV Drug Therapy, 2018.
Glidden, D. and colleagues. Metabolic Effects of Preexposure Prophylaxis With Coformulated Tenofovir Disoproxil Fumarate and Emtricitabine. CID, 2018.
Hill, A. Are new antiretroviral treatments increasing the risks of clinical obesity? 17th European AIDS Conference, 2019.
Mallon, P. and colleagues. Changes in lipids after a direct switch from TDF to TAF. CROI, 2019.
Spinner, C. and colleagues. DISCOVER study for HIV pre-exposure prophylaxis (PrEP): F/TAF has a more rapid onset and longer sustained duration of HIV protection compared with F/TDF. IAS 2019.
Venter, W. and colleagues. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. NEJM, 2019.
—
San Francisco AIDS Foundation receives funding from corporate partners including those in the pharmaceutical industry. Editorial decisions on our blog and website are made independently. For more information about SFAF funding, please refer to our financial and tax documents.