Strong warning against Dovato 2-drug regimen as first-line therapy by San Francisco HIV expert

Historically, HIV treatments have included three or more medications (oftentimes combined in one pill) to keep HIV suppressed and help people living with HIV reach and maintain undetectable viral loads. In the spring of 2019, the U.S. Food and Drug Administration (FDA) approved the complete regimen combo pill Dovato (dolutegravir + lamivudine), manufactured by ViiV Healthcare, giving clinicians for the first time a two-drug option to treat individuals just beginning HIV treatment.
Now, recently updated U.S. Department of Health & Human Services (DHHS) HIV treatment recommendations list a two-drug regimen (dolutegravir + lamivudine) as a first-line recommended therapy. But, Monica Gandhi, MD, MPH, from Zuckerberg San Francisco General Hospital’s Ward 86 gives a strong word of caution due to concerns over drug resistance.
“I’m concerned that this will lead to massive amounts of Dovato use, two-drug therapy, that will bite us down the road,” said Gandhi, who is medical director of Ward 86. “We have lots of experience with three drugs, and there is a concern about resistance with a two-drug regimen.”
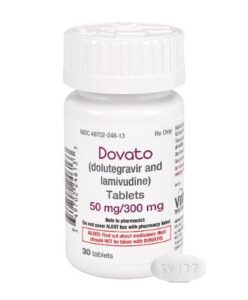
Gandhi explained that lamivudine (3TC), the NRTI in Dovato, has a low barrier to resistance—meaning that it’s easy for a person to develop resistance to the effects of 3TC so that the drug no longer works. When this happens if a person is taking Dovato, the person is effectively on dolutegravir monotherapy. And, people taking only dolutegravir can develop resistance mutations that would rule out future treatment with dolutegravir and, likely, other integrase inhibitors. Dolutegravir monotherapy, Gandhi said, is “a terrible idea.”
“If you lose [develop resistance to] dolutegravir after some time, you’ve just lost the entire first-line class of drugs that we have to treat HIV [INSTIs],” she said.
Drug resistance can be transmitted (i.e., it’s possible for a person who has never taken HIV medications to already have a resistance mutation), so HIV clinicians test for resistance mutations prior to starting therapy. If a person has a resistance mutation, HIV providers can tailor the drug treatment to work around resistance. Resistance can also develop if someone taking HIV medication isn’t adherent to treatment—for instance if they forget to take or aren’t able to take medications every day as prescribed.
For these reasons, Gandhi urges clinicians to consider adherence when prescribing two-drug regimens—knowing that “we’re just not that good” at estimating how adherent a person can be.
“I would be more comfortable at this point giving people a chance to adhere and do well with a three-drug regimen, and then maybe downgrading them to a two-drug regimen if they are adherent,” said Gandhi. “Patients should be aware of the importance of adherence, which is true of any regimen, but particularly true with a two-drug therapy. I wouldn’t want them to miss any doses. And I would want them to talk to their provider about how to take medication, so that they both could be reassured.”
Gandhi said she worries that health care providers who do not specialize in HIV treatment may miss some of these nuances with the new DHHS guidelines—opting to prescribe a two-drug regimen out of concerns over toxicities in regimens with three drugs.
“Young healthy people probably aren’t going to get toxicities with TAF or abacavir (the third drug in the combo pills Biktarvy and Triumeq, respectively). I think it’s interesting that we are talking about two-drug therapy now due to concern about toxicities. For example, NSAIDS (e.g., ibuprofen) can cause renal issues, but we don’t even think twice before putting people on long-term NSAIDS if they’re young and not at risk of renal toxicity. You want to tailor your toxicity concerns to risk factors of that individual. If they have risk factors for cardiovascular disease, you should be concerned about abacavir. If they have risk factors for renal toxicity, you should be concerned about TAF,” said Gandhi
FDA approved Dovato for the initial treatment of HIV based on the results of the GEMINI 1 and 2 studies, which enrolled over 1,400 people starting HIV treatment for the first time. The studies found that 86% of individuals had undetectable viral loads <50 copies after 96 weeks (compared to 89.5% of people taking a three-drug regimen of dolutegravir + TDF/FTC). 85% of participants were men and two-thirds were white.
Although these study results show that dolutegravir + lamivudine can be a successful treatment option for some, Gandhi said that she questions the extrapolation of one phase 3 study to the entire population of people living with HIV.
“People who get into clinical trials are often very rarified populations. They are adherent, they come in for clinical trial visits. They are often white and they are often men. We need some real-world studies, some demonstration projects, that include women and people who may have adherence difficulties,” said Gandhi.
Also new in the DHHS guidelines is a recommendation that HIV treatment be started immediately or as soon as possible after diagnosis, to decrease the time required to achieve viral suppression and reduce risk of HIV transmission, a recommendation Gandhi supports and said she was pleased to see.
The challenges—and opportunities—of two-drug regimens
What are some of the issues with injectables and other two-drug therapies? Find out what you need to know about HIV two-drug regimens. Plus, get an overview of other combinations currently being tested. Read more here.
Dive into the world of drug resistance
Here’s a low-down on HIV drug resistance, including what it is and how you get tested for it. Also, get advice from HIV clinicians on prevention and what to do if you do develop HIV drug resistance.
—
San Francisco AIDS Foundation receives funding from corporate partners including those in the pharmaceutical industry. Editorial decisions on our blog and website are made independently. For more information about SFAF funding, please refer to our financial and tax documents.